
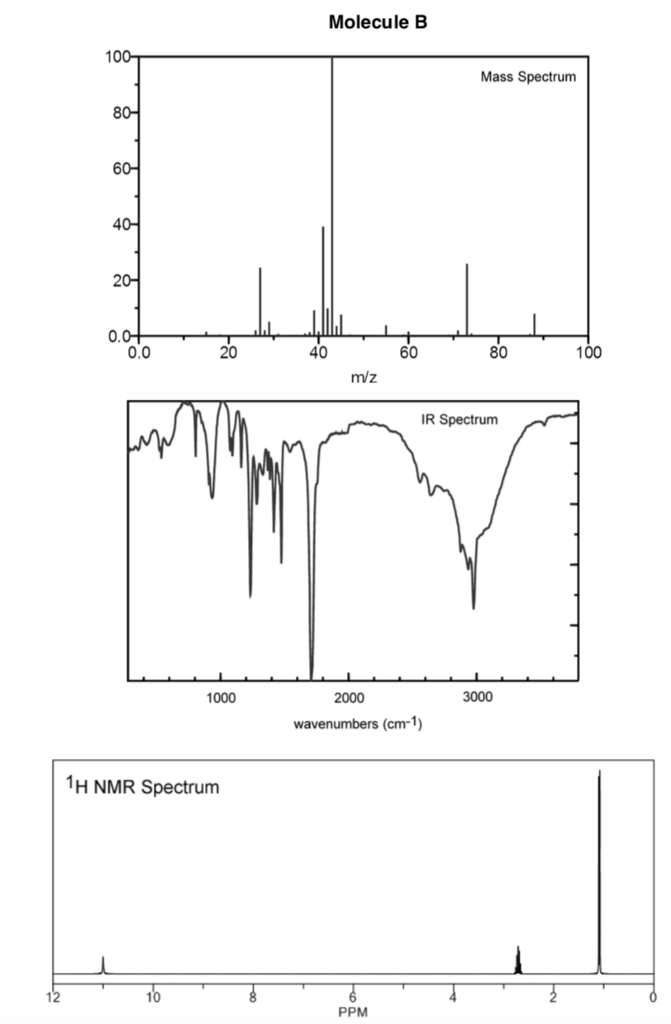
The positions and intensities of the doublet peak also remains constant as a function of pressure and pH. If a protic deuterated solvent is used (e.g., D 2O or CD 3OD), then the NH and OH protons will exchange with the deuterium and the peaks will shrink or disappear 2entirely, since D (H) does not show up in the 1H NMR spectrum. The PF 6 − ion was found to provide a suitable 19F shift and intensity standard for high-pressure spectra because it was chemically inert. broad peaks and usually do not couple with neighboring protons (typically they are broad singlets).
Mestrenova nmr peak broad how to#
I use MestRenova for most of my nmr processing but I havent been able to figure out how to color code individual peaks properly using it.
These peaks broaden and move with pressure in ways that suggest reversible interconversion of borate and fluoroborate species. I have a stacked 1H NMR spectra of a time monitored reaction and I want to color code specific peaks of the starting materials and products. This is a standard reference point with the signal set exactly at 0 ppm and y ou can ignore it when analyzing an NMR spectrum. Additionally, there is a single peak that is assignable to H 3BO 3 o(aq) and B(OH) 4 −(aq) in rapid-exchange equilibria. The only peak that comes before saturated C-H protons is the signal of the protons of tetramethylsilane, (CH3) 4 Si, also called TMS. Peaks in the 11B spectra at pressure could be assigned to the BF 4 −(aq) and BF 3OH −(aq) species. At pressure, peaks in the 19F spectra were clear and assignable to the BF 4 −(aq), F −(aq) and BF 3OH − (aq) ions, and these aqueous complexes varied in signal intensity with pressure and time, for each solution. 2 Examples would include Brukers CONVDTA command and mNovas. iNMR not only contains all the above things, but also a Spotlight-based search module, a Chessboard-like plot mode, and some nice managers (like the Overlays manager and the J-manager). difficult (broad and/or noisy) for the automatic peak picking algorithm to detect. Correspondingly, NMR spectra of 19F- and 11B were collected on aqueous HBF 4-NH 4PF 6 solutions to pressures up to 2.0 GPa. I say unfinished because many important things are missing: simulation of spin systems, simulation of chemical exchange, peak deconvolution, 3-D NMR. Fluoride solutions were chosen for study because 19F couples to other nuclei in the solutions ( 31P and 11B) in ways that make peak assignments unequivocal. We therefore designed an NMR probe that can be used to study aqueous solutions at gigapascal pressures. Aqueous geochemistry could be extended considerably if nuclear-magnetic resonance (NMR) methods could be adapted to study solutions at elevated temperatures and pressures.


 0 kommentar(er)
0 kommentar(er)
